What is Phentermine?
Phentermine is an amphetamine-like prescription appetite suppressant used for weight loss.
First approved in 1959, phentermine is the most-prescribed weight loss medication in the United States to treat together with diet and exercise obesity (
Mechanism of Action
Phentermine activates the central nervous system and stimulates the release of certain neurotransmitters like dopamine, epinephrine (adrenaline), and norepinephrine (noradrenaline).
As a result, the central nervous system induces a “fight or flight”-like reaction, which causes increased heart rate, sweating, lack of appetite, and extra energy.
Active Ingredient
In the United States and Latin America, most phentermine-based weight loss pills contain phentermine hydrochloride as their main ingredient.
However, phentermine resin-based pills are still available in other parts of the world (such Ionamin and Duromine).
Get your FREE Phentermine Guide!
Learn more about popular brands, dosage, side effects, results, and weight loss alternatives.
Who Takes It
Phentermine is a class IV controlled substance, so it is only available under a doctor’s prescription (
This weight loss medication is an option for patients with a body mass index (BMI) greater than or equal to 30 kg/m2, which puts them in the “obese” range.
Phentermine is also available to individuals with a BMI of 27-29.9 kg/m2 plus a weight-related condition like type 2 diabetes, high cholesterol, or controlled hypertension (
However, this medication is not appropriate for all overweight and obese patients. There are many contraindications for phentermine use, including, but not limited to, a history of cardiovascular disease, history of addiction, history of agitated states, or current/recent use of certain medications or supplements (

Treatment Duration
Phentermine is intended as a short-term treatment as it is potentially habit-forming. It should only be taken for three months at a time (
This recommendation is based on the original drug trials in which participants were monitored over 12 weeks, and the safety of more prolonged medication use was not assessed.
However, some doctors will prescribe phentermine for more extended periods, or prescribe it several times within a specified period with month-long breaks between each period. Such a treatment plan must be evaluated on a case-by-case basis.
Dosage
Phentermine weight loss pills are available in both tablet and capsule form, in doses ranging from 8 – 37.5 milligrams.
The lowest-dose tablets (Lomaira 8mg) can be taken up to three times per day, while higher-dose tablets and capsules can only be taken 1-2 times per day. The exact phentermine dosage and schedule varies by individual.
Morning
Morning
Morning
The maximum dose of phentermine hydrochloride is 37.5 mg per day.
Adverse Reactions
Phentermine is a highly-effective weight loss medication, but it also has a long list of potential side effects.
Serious reactions to phentermine, which may be symptoms of a potentially-fatal heart or lung problem, include (
- Chest pain
- Irregular (fluttering) or pounding heart rate
- Shortness of breath
- Lightheadedness or fainting
- Swelling of the ankles, legs, or feet
- Stroke-like symptoms, such as slurred speech or one-sided weakness
- Extreme happiness or sadness
However, most people experience only side effects. The most commonly reported reactions are dry mouth, insomnia, constipation, and headache (
Most patients report fewer side effects as the treatment progresses. Lower-dose pills or alternate schedules can also help alleviate the bothersome reactions faster.
History
Despite the growing necessity for pharmaceutical weight loss options, many doctors and potential patients remain skeptical of phentermine due to its troubled past.
During the 1980-the 90s, doctors commonly prescribed an off-label combination of fenfluramine (fen) and phentermine (Phen) for weight loss (
However, in 1997, the Mayo Clinic reported that “fen-phen” caused heart valve irregularities and primary pulmonary hypertension in some patients. Fenfluramine, the likely culprit, was withdrawn from the market soon after (
Phentermine has remained available with a warning on the package insert (
Frequently Asked Questions
Is Phentermine an Amphetamine?
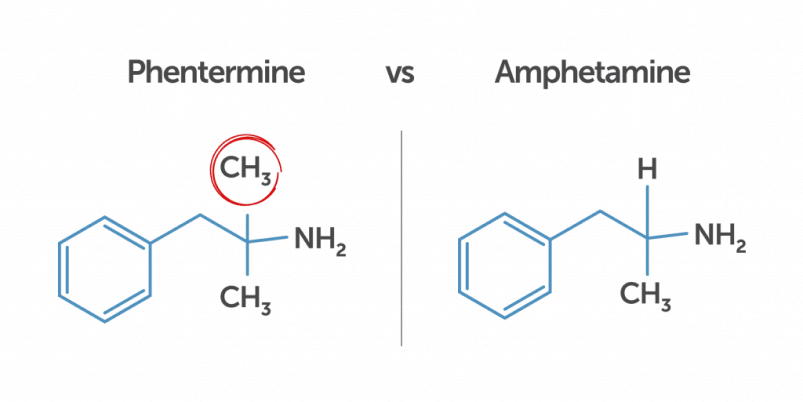
No, phentermine is not an amphetamine.
However, it does have a chemical structure similar to amphetamines, so phentermine weight loss pills can cause a false positive on 5-panel drug screens.
Is Phentermine Safe?
Phentermine is an FDA-approved medication and is generally safe when taken as prescribed.
Phentermine, like all other medications, can cause side effects. However, when doctors prescribe a weight loss drug such as phentermine, it is because the benefits of losing weight to reach a healthy BMI outweigh the possible side effects.
- U.S. Food and Drug Administration. (2012). Adipex-P (phentermine hydrochloride) capsules label [Brochure].
- National Center for Biotechnology Information. PubChem Compound Database: CID=4771 (Phentermine).
- Hendricks, E. J., Rothman, R. B., & Greenway, F. L. (2009). How Physician Obesity Specialists Use Drugs to Treat Obesity. Obesity, 17(9), 1730-1735. doi:10.1038/oby.2009.69
- Rueda-Clausen, C.F., Padwal, R.S., Sharma, A.M. (2013). New pharmacological approaches for obesity management. Nature Reviews Endocrinology, 9(8):467-478.
- WebMD. (2019). Phentermine Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing.
- CardioSmart: American College of Cardiology. (2011, November 21). Phentermine.
- Members of “Losing Weight with Phentermine” Support Group on Facebook & Phentermine.com Forum. (2019, March 13). [User Report of Common Phentermine Side Effects]. Unpublished raw data.
- Weintraub M, Hasday JD, Mushlin AI, Lockwood DH. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch Intern Med. 1984;144(6):1143-1148.
- Connolly, H. M., Crary, J. L., McGoon, M. D., Hensrud, D. D., Edwards, B. S., Edwards, W. D., & Schraff, H. V. (1997). Valvular Heart Disease Associated with Fenfluramine–Phentermine. New England Journal of Medicine, 337(24), 1772-1776. doi:10.1056/nejm199708283370901
- Centers for Disease Control and Prevention. (1997). Cardiac Valvulopathy Associated With Exposure to Fenfluramine or Dexfenfluramine: US Department of Health and Human Services Interim Public Health Recommendations, November 1997. JAMA: The Journal of the American Medical Association, 278(21), 1729. doi:10.1001/jama.1997.03550210025016
- Teva Pharmaceuticals USA, Inc. (2017). Adipex-P: Highlights of Prescribing Information
- Buchwald, H., Cowan, G. S., & Pories, W. J. (2007). Surgical management of obesity. Philadelphia, PA: Elsevier.
Learn about Phentermine.
