Qsymia: A Prescription Weight Loss Medication
Qsymia is a medication prescribed for weight loss that combines phentermine HCl and topiramate extended-release. Approved by the FDA in 2012, it offers long-term obesity management compared to phentermine alone but has additional potential side effects.
What is Qsymia?
Qsymia contains phentermine HCL (Adipex) and topiramate extended-release (Topamax). Phentermine acts as a central nervous system stimulant, reducing hunger and boosting energy by triggering a “fight or flight” response. Topiramate, an anti-seizure medication, aids in weight loss by increasing feelings of fullness, making food less appealing, and boosting caloric expenditure.
This medication is prescribed for adults with a body mass index (BMI) over 30 kg/m² or those with a BMI over 27 kg/m² who have weight-related complications like type 2 diabetes or high blood pressure. Unlike traditional phentermine pills, which are limited to 12 weeks of use, Qsymia is approved for long-term use, up to three years, when combined with a healthy diet and regular exercise.
Contraindications
While generally safe when used as directed, Qsymia is not suitable for everyone. It should not be used by individuals who are pregnant, have glaucoma or hyperthyroidism, have a history of reaction to sympathomimetic amines, or are using monoamine oxidase inhibitors (MAOIs).
Additional precautions include informing your doctor of allergies, heart disease, high blood pressure, mental health conditions, liver or kidney disease, or other health concerns. It’s also crucial to discuss all current medications with your healthcare provider to avoid potential drug interactions.
Dosage and Administration
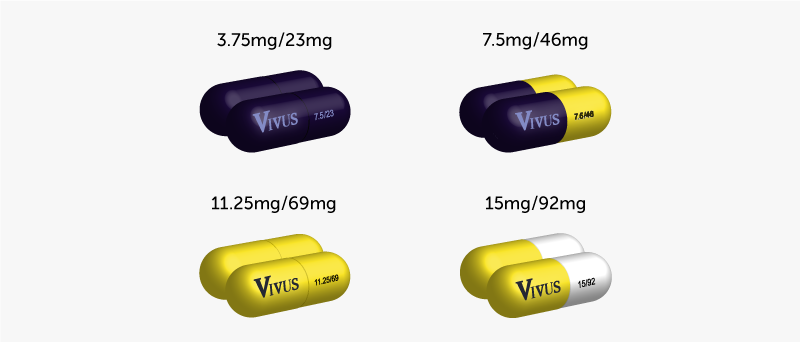
Qsymia is available in four dosage strengths, each combining different amounts of phentermine and topiramate. The available strengths are:
- 3.75 mg/23 mg: This is the starting dose, marked with a purple cap and body imprinted with “VIVUS” and “3.75/23”.
- 7.5 mg/46 mg: The next step in the dosage escalation, featuring a purple cap and a yellow body imprinted with “VIVUS” and “7.5/46”.
- 11.25 mg/69 mg: Used for further dosage escalation if needed, identified by a yellow cap and body imprinted with “VIVUS” and “11.25/69”.
- 15 mg/92 mg: The highest dosage strength, with a yellow cap and body imprinted with “VIVUS” and “15/92”.
These extended-release capsules are designed to provide a controlled release of the medication throughout the day, optimizing its effectiveness while minimizing side effects.
It’s essential to follow your doctor’s instructions precisely and not to alter the dosage without medical advice. If a dose is missed, skip it and continue with the next dose as scheduled. Do not double up on doses.
Side Effects Specific to Qsymia
There are side effects specific to Qsymia that are not typically associated with phentermine alone.
Common Side Effects
- Changes in Taste: Qsymia can cause dysgeusia, leading to a metallic taste or a general loss of taste. This is not commonly reported with phentermine alone.
- Tingling Sensations: Another unique side effect of Qsymia is paraesthesia, which involves numbness or tingling in the hands, arms, feet, or face. This is due to the topiramate component of the medication.
Serious Side Effects
- Cognitive Impairment: Qsymia can cause mental issues such as difficulties with concentration, memory, and speech. These cognitive side effects are attributed to topiramate and are not typically seen with phentermine alone.
- Metabolic Acidosis: Qsymia can lead to metabolic acidosis, a condition characterized by increased acid levels in the blood. This can result in symptoms like fatigue, loss of appetite, and changes in heartbeat. If left untreated, it can cause serious complications such as brittle bones and kidney stones.
- Severe Skin Reactions: Qsymia has been associated with severe skin reactions, including Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. These conditions involve painful rashes and blisters and can be life-threatening if not treated promptly.
- Increased Heart Rate: While phentermine alone can increase heart rate, combining the drug with topiramate can exacerbate this effect. Patients are advised to monitor their heart rate and consult their healthcare provider if they experience a racing or pounding heart while at rest.
- Suicidal Thoughts or Actions: The topiramate component in Qsymia can lead to suicidal thoughts or actions, a severe side effect that requires immediate medical attention. This is not a common side effect of phentermine alone.
How to Get a Prescription
To obtain Qsymia, schedule an appointment with your primary care doctor or a weight loss specialist. This medication is a schedule IV-controlled substance and requires a prescription.
Where to Fill Your Prescription
Prescriptions can be filled at most major pharmacies in the United States or through certified mail-order pharmacies like Walgreens, Walmart, and Kaiser Permanente.
How Much Does It Cost?
The cost for a 30-day supply ranges from approximately $187 to $220 before discounts. Most insurance plans do not cover weight loss medications; however, VIVUS, Inc. offers a discount program that caps the monthly cost at $98 and includes home delivery.
User Reviews
Overall, user reviews are mixed. Some users report significant weight loss and satisfaction with minimal side effects, while others experience severe side effects and minimal weight loss.
Here are some reviews from recent users:
Only been taking Qsymia for a little over 2 weeks. So happy with it! I keep water nearby all the time because I’m ALWAYS thirsty, but no other side effects. Actually, have to remind myself to eat… So far, I’ve lost 7 pounds. Can’t wait to keep watching the weight fall off!
Becky
Not worth it… Never did anything for me. Only lost 13 pounds in 3 MONTHS and the side effects were terrible, especially the first month. Better to do weight watchers or something like that.
Raquel
Started this medication on September 6 and weighed in at 176 lbs. Today is November 3 current weight is 153 lbs – no workouts/gym. I only take one pill daily in the morning. I will be getting my last fill on Monday, very happy with this medication. The only side effects were the first 3 days; I experienced insomnia.
Perksta
Conclusion
In summary, while Qsymia can be an effective tool for weight loss, it requires careful consideration and medical supervision to navigate its potential side effects. Always consult your healthcare provider to determine if Qsymia is the right choice for your weight management plan.

